ocrevus start form pdf
OCREVUS Start Form for ocrelizumab Who May See and Use My PII I authorize Genentech andor Genentech Patient Foundation to i use my PII for the purpose of facilitating my access. Is this a new start or continuation of therapy.

Controversy On The Treatment Of Multiple Sclerosis And Related Disorders Positional Statement Of The Expert Panel In Charge Of The 2021 Dgn Guideline On Diagnosis And Treatment Of Multiple Sclerosis Neuromyelitis Optica
It must be completed by the provider.

. Prior Authorization Form for. Send it via fax. Helping schedule and prepare patients for their infusions.
If your patient has already begun treatment with drug samples of Ocrevus please choose new start of therapy. Ocrevus ocrelizumab Vials are diluted in NS Initial dose two infusions Note. O When possible you should receive any non-live vaccines at least 2.
There is a pregnancy exposure registry that monitors pregnancy and fetalneonatalinfant outcomes in women exposed to OCREVUS during pregnancy. Si desea suscribirse a programas de marketing y. Sample infusion referral form Please confirm compliance.
Once we have both. Start at 40mlhr increasing by 40mlhr every 30 min to a max rate of 200mlhr. To a final concentration of 12mgmL.
The OCREVUS Start Form is required for enrollment in OCREVUS Access Solutions. Ocrevus ocrelizumab 02-micron filter must be used during infusion Initial dosing. Loading doses must be administered in a controlled infusion site.
_____ Current Patient New Patient Need by date. Welcome Guide - OCREVUS ocrelizumab. Sign a printed form and fax or mail it to us or give it to your doctors office to do so Your doctor also has to fill out a form called the OCREVUS Start Form.
300mg10mL SDV to a final concentration. Physicians are encouraged to. According to immunization guidelines live or live-attenuated vaccines should be administered at least 4 weeks prior to initiation of.
Locating appropriate infusion sites such as infusion centers HCP offices or home infusion providers based on patients clinical. Duration should be at least 35 hrs. Si desea suscribirse a OCREVUS Access Solutions complete la SECCIÓN 1 y coloque firma y fecha en la SECCIÓN 1A de la página 4.
Ocrevus 600mg500ml IV every 6 months 24 weeks. Relapsing forms of multiple sclerosis MS to include clinically isolated syndrome relapsing-remitting disease and. Infuse 300mg IV in 250ml NS over a minimum of 25 hours on day 0 and 14.
The form includes patient insurance and. Ocrevus ocrelizumab Vials are diluted in NS Subsequent doses one infusion 300mg10mL SDV. Relapsing forms of multiple sclerosis MStoincludeclinically isolated syndrome relapsing-remitting disease and.
OCREVUS START FORM Y Medical Associates Fax Referral To. OCREVUS is aCD20-directed cytolytic antibody indicated for the treatment of. Every 6 months infuse 600mg in 500mL of 09 NS.
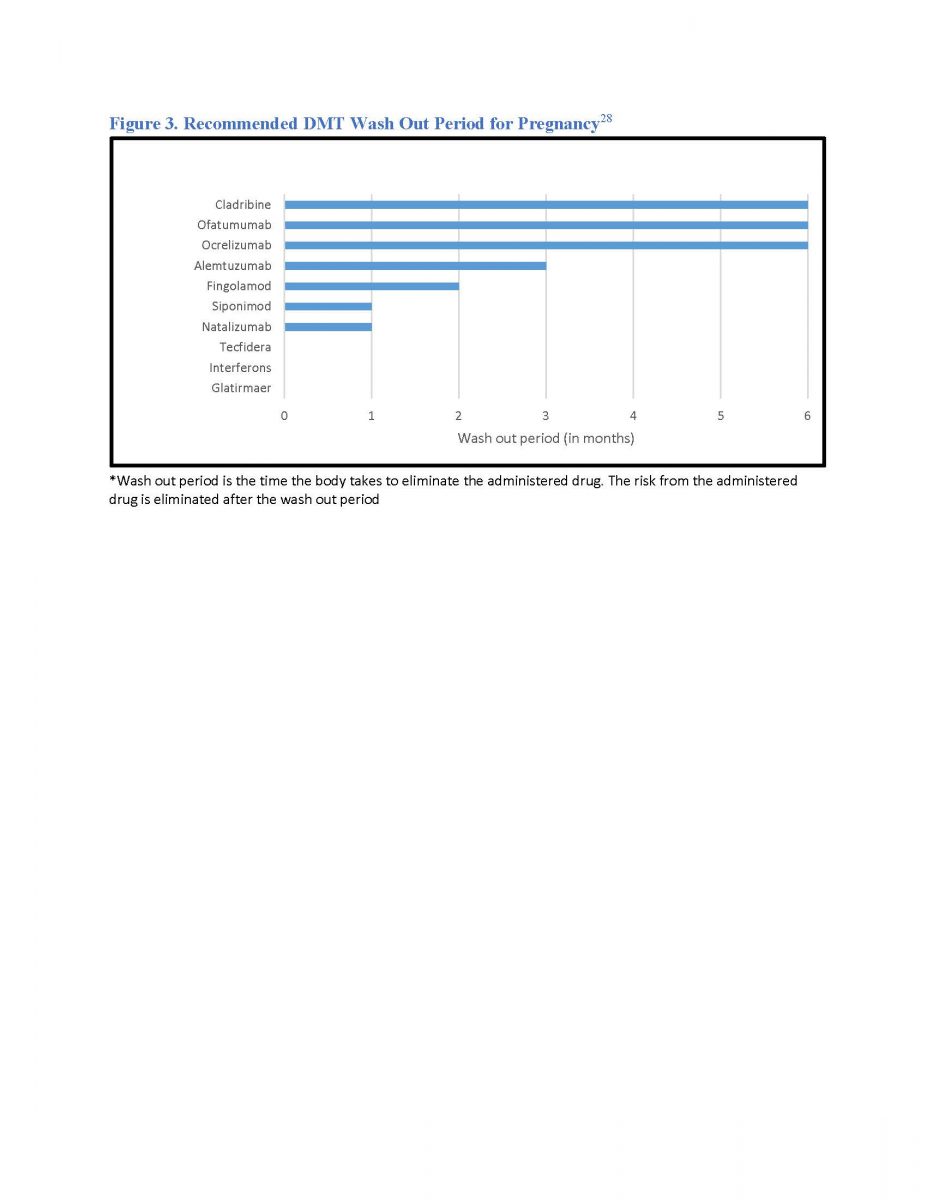
Multiple Sclerosis Evoking The Pharmacy Team S Complete Potential Uconn School Of Pharmacy

How To Switch Disease Modifying Treatments In Multiple Sclerosis Guidelines From The French Multiple Sclerosis Society Sfsep Sciencedirect
Ocrevus Side Effects What They Are And How To Manage Them
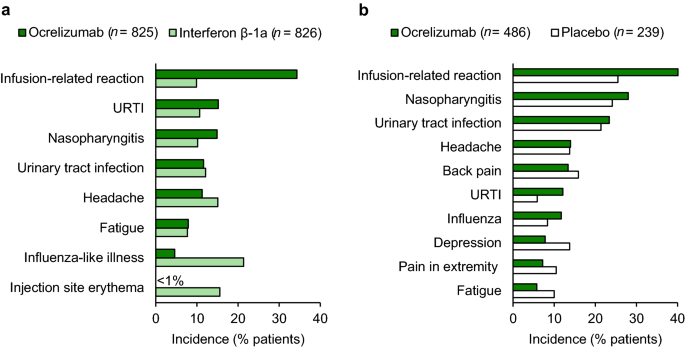
Ocrelizumab A Review In Multiple Sclerosis Springerlink

Ocrevus Ocrelizumab Results For Rms Relapsing Ms

Safety Results Of Administering Ocrelizumab Per A Shorter Infusion Protocol In Patients With Primary Progressive And Relapsing Multiple Sclerosis Multiple Sclerosis And Related Disorders

Pregnancy And Ms Choices Booklet Ms Uk
Pdf Ocrelizumab A New Milestone In Multiple Sclerosis Therapy

Multiple Sclerosis Disease Modifying Therapies The Pharmaceutical Journal
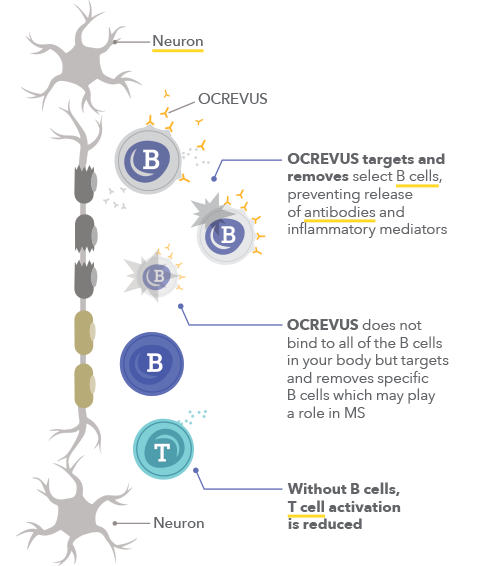
How Does Ocrevus Work Get On With Life

Ocrevus Side Effects Cost Uses And More
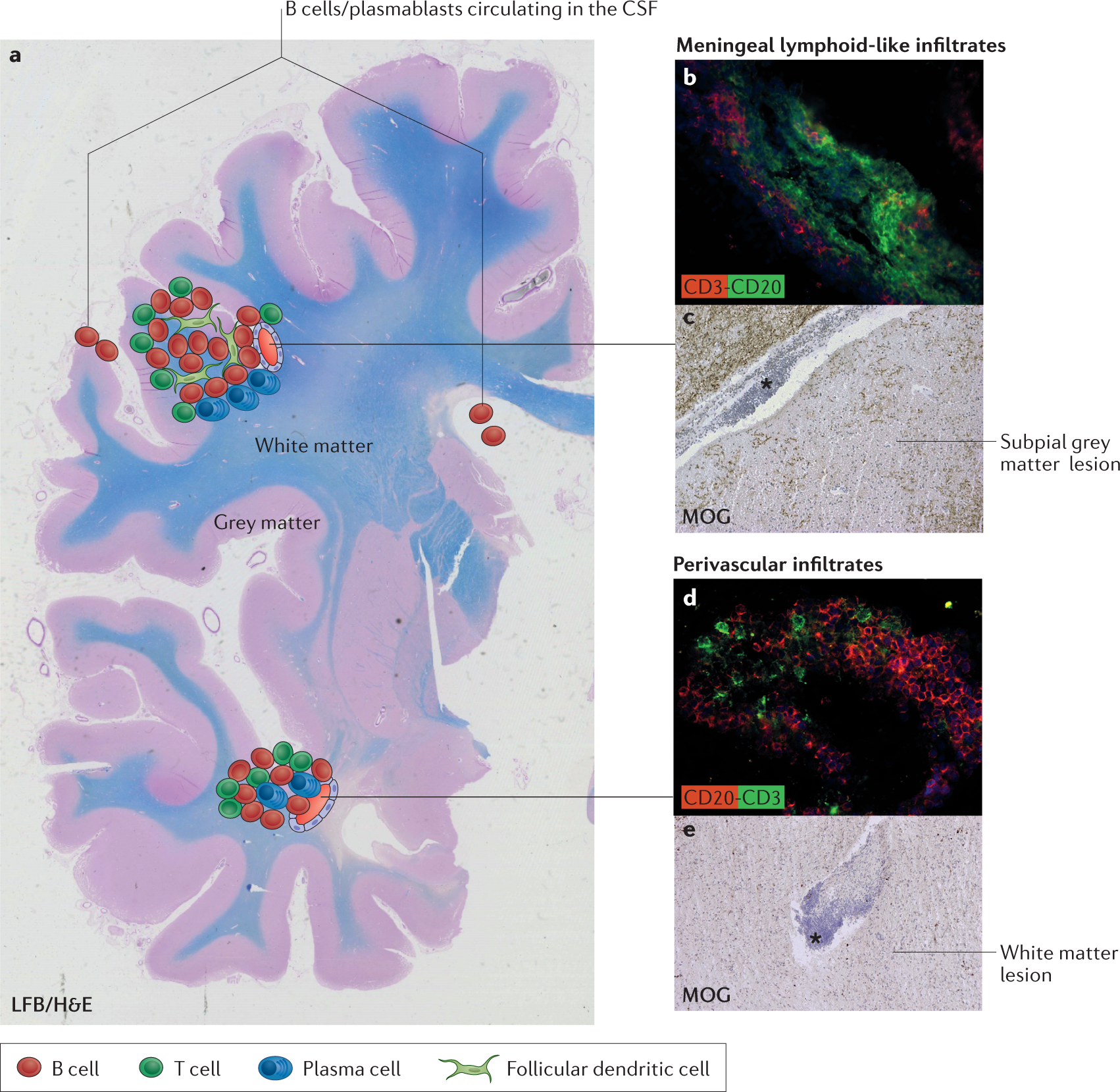
B Cells In Multiple Sclerosis From Targeted Depletion To Immune Reconstitution Therapies Nature Reviews Neurology

Efficacy And Safety Of Ocrelizumab In Patients With Relapsing Remitting Multiple Sclerosis With Suboptimal Response To Prior Disease Modifying Therapies A Primary Analysis From The Phase 3b Casting Single Arm Open Label Trial Vermersch

Frontiers Ocrelizumab In Multiple Sclerosis A Real World Study From Spain

Ocrelizumab Infusion Experience In Patients With Relapsing And Primary Progressive Multiple Sclerosis Results From The Phase 3 Randomized Opera I Opera Ii And Oratorio Studies Multiple Sclerosis And Related Disorders
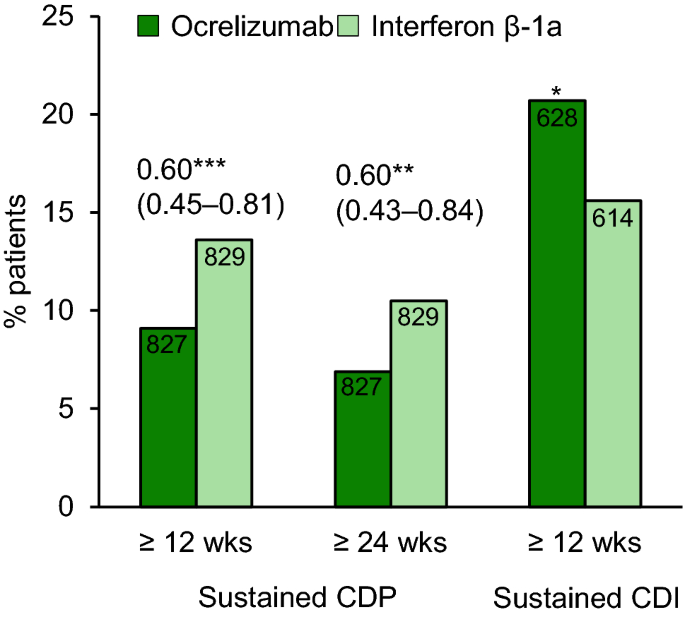
Ocrelizumab A Review In Multiple Sclerosis Springerlink
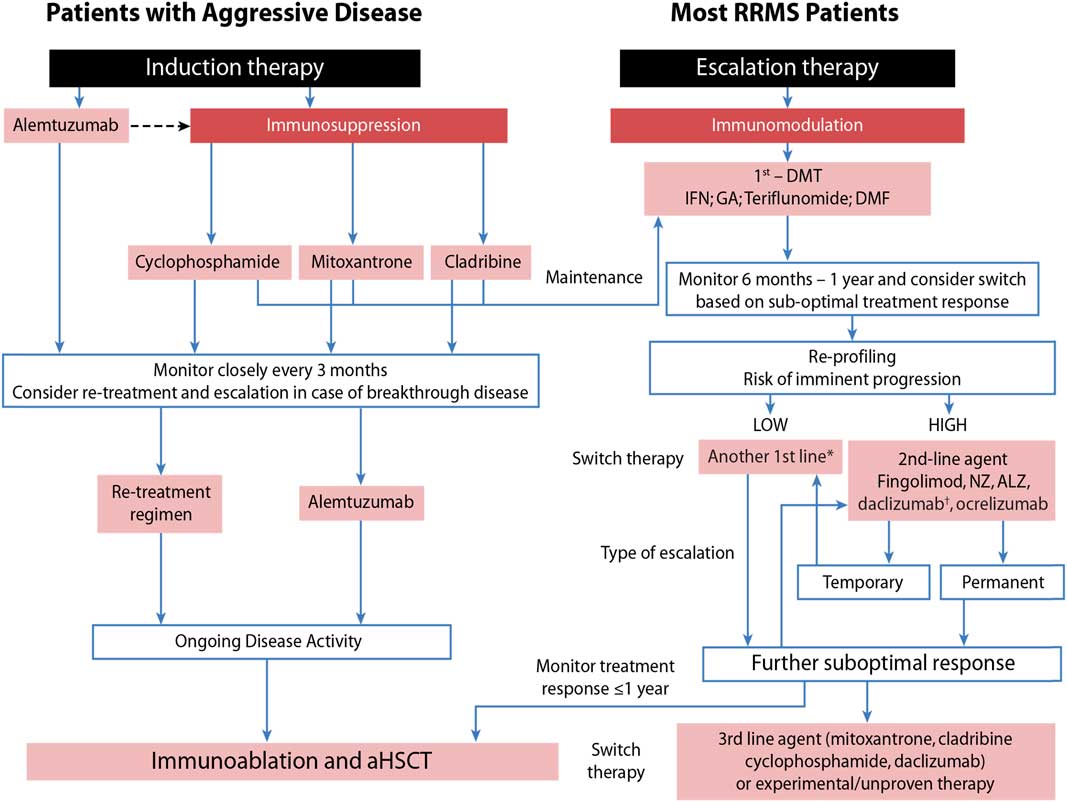
Managing Multiple Sclerosis Treatment Initiation Modification And Sequencing Canadian Journal Of Neurological Sciences Cambridge Core

Roche Gets A Boost From Covid 19 Drug Actemra But Ocrevus Lags As Patients Prep For Boosters Fierce Pharma